|
问题讨论与思考
|
|
 |
|

|
|
|
| 烯烃的羟汞化脱汞:还原脱汞机理* |
| 杨占会**, 许家喜 |
| 北京化工大学化学学院 北京 100029 |
|
| Alkene Oxymercuration-Demercuration: Detailed Mechanism for Demercuration |
| YANG Zhan-Hui**, XU Jia-Xi |
| College of Chemistry, Beijing University of Chemical Technology, Beijing 100029, China |
|
|
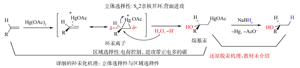
摘要:介绍了不同时期提出的还原脱汞机理,包括Bordwell和Douglass的协同机理、Pasto和Gontarz的自由基笼机理、Whitesides和Filippo的自由基链机理和Quirk和Whitesides等人完善的自由基链机理。对还原脱汞机理的介绍完善了现行教科书中的机理,有利于学生学习完整的有机化学知识和机理,培养正确的有机化学研究理念。
|
|
| 关键词: 羟汞化,
还原脱汞,
机理,
自由基笼,
自由基链
|
|
|
| 基金资助:*“有机化学”和“中级有机化学”一流课程建设项目 |
|
通讯作者:
**E-mail: zhyang@mail.buct.edu.cn
|
| 引用本文: |
|
杨占会, 许家喜. 烯烃的羟汞化脱汞:还原脱汞机理*[J]. 化学教育(中英文), 2023, 44(2): 117-122
|
|
| [1] |
吕萍, 王彦广. 中级有机化学——反应与机理. 北京: 高等教育出版社, 2015: 44-45
|
| [2] |
古练权, 汪波, 黄志纾, 等. 有机化学. 北京: 高等教育出版社, 2008: 398-399
|
| [3] |
鲁崇贤, 杜洪光. 有机化学. 2版. 北京: 科学出版社, 2009: 8, 170
|
| [4] |
魏荣宝. 高等有机化学. 北京: 高等教育出版社, 2007: 232
|
| [5] |
伍越寰, 李伟昶, 沈晓明. 有机化学. 2版. 合肥: 中国科学技术大学出版社, 2002: 64-65
|
| [6] |
刘庆俭. 有机化学. 上海: 同济大学出版社, 2018: 104-105
|
| [7] |
何树华, 张淑琼, 何德勇. 中级有机化学. 北京: 化学工业出版社, 2010: 115
|
| [8] |
史达清, 赵蓓. 有机化学. 北京:高等教育出版社, 2019: 110-111
|
| [9] |
邢其毅, 裴伟伟, 徐瑞秋, 等. 基础有机化学. 4版. 北京: 北京大学出版社, 2016: 306, 324
|
| [10] |
McMurry J. Organic Chemistry. 9th ed. Boston: Cengage Learning, 2016: 229-230
|
| [11] |
Wade L G Jr. Organic Chemistry. 8th ed. Glenview: Pearson Education, Inc., 2013: 340-343
|
| [12] |
Brown W H, Iverson B L, Anslyn E V, et al. Organic Chemistry. 8th ed. Boston: Cengage Learning, 2018: 262-264
|
| [13] |
Carey F A, Giuliano R M. Organic Chemistry. 10th ed. New York: McGraw-Hill Education, 2017: 319-320
|
| [14] |
Klein D. Organic Chemistry. 2nd ed. Hoboken: John Wiley Sons, Inc., 2015: 420-422
|
| [15] |
Clayden J, Greeves N, Warren S. Organic Chemistry. 2rd ed. New York: Oxford University Press Inc., 2012: 444
|
| [16] |
Okuyama T, Maskil H. Organic Chemistry: A Mechanistic Approach. Oxford: Oxford University Press, 2014: 322-323
|
| [17] |
Solomon T W G, Fryhel C B, Snyder S A. Organic Chemistry. 12th ed. Hoboken: John Wiley & Sons, Inc., 2008: 349
|
| [18] |
Vollhardt P K, Schore N. Organic Chemistry: Structure and Function. 8th ed. New York: W. H. Freeman and Company, 2018:544-546
|
| [19] |
Klein D. Klein's Organic Chemistry. global ed. Singapore: John Wiley & Sons, Inc., 2018: 406-407
|
| [20] |
Bordwell F G, Douglass M L. Journal of the American Chemical Society, 1966, 88(5): 993-999
|
| [21] |
Dessy D E, Grannen Jr E. Journal of the American Chemical Society, 1961, 83(19): 3953-3958
|
| [22] |
Pasto D J, Gontarz J A. Journal of the American Chemical Society, 1969, 91(3): 719-721
|
| [23] |
Gray G A, Jackson W R, Chambers V M A. Journal of the Chemical Society C: Organic, 1971: 200-204
|
| [24] |
Cristol S J, Brindell G D, Reeder J A. Journal of the American Chemical Society, 1958, 80(3): 635-640
|
| [25] |
Whitesides G M, Filippo J S Jr. Journal of the American Chemical Society, 1970, 92(22): 6611-6624
|
| [26] |
Kuivia H G. Accounts of Chemical Research, 1968, 1(10): 299-305
|
| [27] |
Quirk R P. Journal of Organic Chemistry, 1972, 37(22): 3554-3555
|
| [28] |
Kaplan L. Journal of the American Chemical Society, 1966, 88(19): 4531
|
| [29] |
Carlsson D J, Ingold K U. Journal of the American Chemical Society, 1968, 90(25): 7047-7055
|
| [30] |
North A M. Quarterly Reviews, Chemical Society, 1966, 20(3): 421-440
|
| [31] |
Hill C L, Whitesides G M. Journal of the American Chemical Society, 1974, 96(3): 870-876
|
| [32] |
Quirk R P, Lea R E. Tetrahedron Letter, 1974, 15(22): 1925-1928
|
| [33] |
Quirk R P, Lea R E. Journal of the American Chemical Society, 1976, 98(19): 5973-5978
|
| [34] |
Lal D, Griller D, Husband S, et al. Journal of the American Chemical Society, 1974, 96(20): 6355-6357
|
| [35] |
Groves J T, Ma K M. Journal of the American Chemical Society, 1974, 96(20): 6527-6529
|
| [36] |
Zard S Z. Organic Letter, 2017, 19(6): 1257-1269
|
| [37] |
Kitching W. Comprehensive Organic Synthesis, 1991, 7: 613-639
|
|
|
|